Today’s blog is written by guest bloggers Morgan Peters1,2, Jack Ballantyne1,3, and Erin Hanson1,3
1 National Center for Forensic Science, University of Central Florida, Orlando, FL USA, 2 Graduate Program in Chemistry, University of Central Florida, Orlando, FL USA ,3 Department of Chemistry, University of Central Florida, Orlando, FL USA
Delayed reporting of sexual assault is very common and can be detrimental to obtaining the autosomal STR (aSTR) profile from a semen donor. Following standard aSTR analysis methods, it is typically not possible to obtain a male profile when the post-coital interval reaches 48-72 hours. This is not due to the absence of sperm cells, but rather the abundance of victim epithelial cells analytically overwhelming the limited number of sperm. One common approach to address this issue is to perform Y-STR analysis in place of aSTR analysis. While Y-STRs are very useful and should not be discounted, they do not hold the same statistical weight as aSTR analysis. Our approach to addressing the issues with aSTR analysis of late reported samples is to apply a modified version of our previously established micromanipulation methodology, in which rare individual sperm cells are manually collected into lysis mix and then directly amplified – eliminating the need for a quantification step.
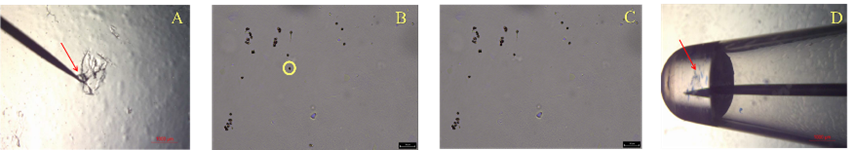
A sperm pellet created from a semen swab was resuspended in nuclease free water. A fresh swab was dipped into the resuspension and then rolled onto a Gel-Film slide. Dried slides were stained with Nuclear Fast Red solution. A small amount of 3M water soluble adhesive was collected on the tip of a tungsten needle and touched to the cell to collect it (Figure 1A-C). The needle tip was then placed into 1 µL Casework Direct lysis mix until the adhesive was observed to have solubilized (Figure 1D). Samples were lysed for 30 minutes at 70°C. PCR mix was then added directly to the lysis mix (GlobalFiler™ Express – 5 µL, Yfiler™ Plus – 4 µL) with all samples undergoing 32 PCR cycles.
For single sperm aSTR analysis, the maximum possible allele recovery is 50% since each sperm only contains one copy of each chromosome. We achieved up to a 47% allele recovery from single sperm, with an average allele recovery of 20% (n=25). Using Y-STR analysis, it should be possible to obtain a full profile from a single sperm. However, only sperm containing a Y chromosome (i.e. approximately 50% of randomly recovered sperm) will produce any results. In this study, allele recovery was observed in 45% of single sperm Y-STR samples (n=175). From these samples the average allele recovery was 18%, with a maximum allele recovery of 92%.
Analysis was also performed using 2-5 cells (n=25), 10 cells, and 25 cells (n=10). As expected, for both aSTR and Y-STR samples, as the cell number was increased the percentage of alleles recovered also increased. A full aSTR profile was produced with as few as five sperm and a full Y-STR profile with as few as two cells. Average aSTR recoveries were, in order of increasing cell quantity, 47%, 54%, 64%, 73%, 90%, and 99%. Following the same pattern, Y-STR recoveries were 49%, 50%, 62%, 64%, 77%, and 96%.
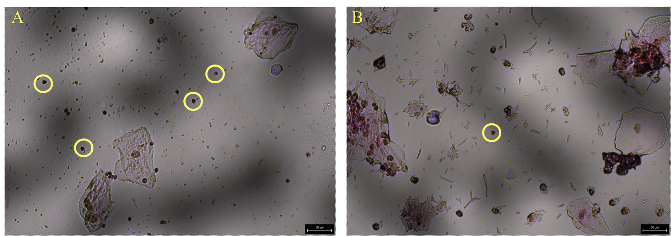
This methodology was also used to perform aSTR analysis on sperm cells collected from epithelial/semen mixtures (Figure 2). Sperm cells that were not adhering to epithelial cells were targeted for collection. Replicates (n=10) were tested in quantities of 3-5 cells, resulting in average allele recoveries of 49%, 67%, and 73%, respectively. Maximum allele recoveries of 76%, 97%, and 90% were obtained. The results produced indicated that the samples were single source, with the profiles confirmed to have originated from the semen donor (comparison to reference profiles).
This project is currently being expanded to evaluate the use of our lab’s previously established Y-Chromosome-Targeted Pre-Amplification (Y-TPA) methodology on single sperm samples. Additionally, we will continue to evaluate more simulated sexual assault samples with the goal of working towards genuine post-coital samples.
The methodology described here could significantly improve the ability to obtain an offender DNA profile from a late reported sexual assault. The current recommended time frame for evidence collection of vaginal samples is up to 120 hours (5 days). We are hopeful with the use of the approach described here, in which the few remaining sperm cells in a sample are isolated for direct analysis, that the recommended interval for sample collection can be extended in the future as these late reported samples could potentially lead to highly probative offender profiles being recovered.
We would like to acknowledge all of the anonymous donors who provided samples for this study. Portions of this work were supported by Award No. 2018-NE-BX-001, awarded by the National Institute of Justice, Office of Justice Programs, U.S. Department of Justice. The opinions, findings and conclusions or recommendations in this publication are those of the authors and do not necessarily reflect those of the Department of Justice.
WOULD YOU LIKE TO SEE MORE ARTICLES LIKE THIS? SUBSCRIBE TO THE ISHI BLOG BELOW!
SUBSCRIBE NOW!